Which of the Following Statements About Redox Reactions Is False
B The reaction will give a single product of C 2 H 5 Cl. Carboxy-H 2 DCFDA mixed isomers chloromethal-H 2 DCFDA mixed isomers DAF-FM diacetate H 2 DCFDA HPF OxyBURST Green and Peroxy-Fluor 2 PF2 or an equivalent volume of dH 2 O our no-dye control.
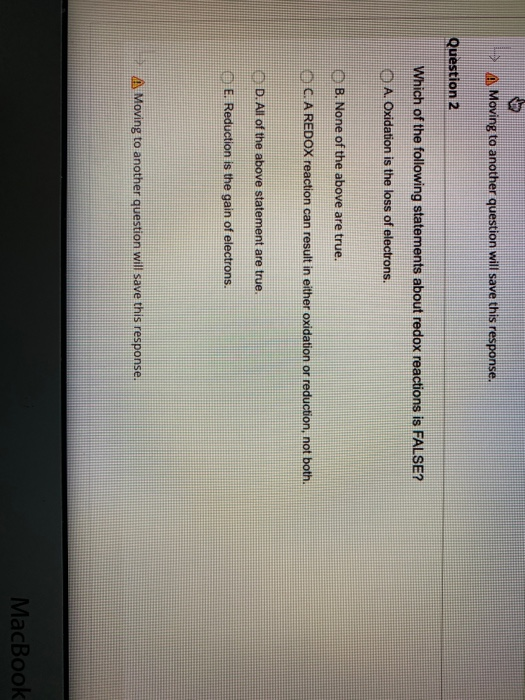
Solved Moving To Another Question Will Save This Response Chegg Com
Which of the following statements is not true of most cellular redox reactions.

. D The reaction can. Hydrazine is highly toxic unless handled in solution as for example hydrazine hydrate NH 2 NH 2 xH 2 OAs of 2015 the world hydrazine hydrate market amounted to 350 million. Hydrazine is mainly used.
Proteins assist the passage of materials into the cellb. Which of the following statements is FALSE regarding the reaction between Cl 2 and C 2 H 6. If soluble in water then the solutions are usually neither strongly acidic nor strongly basic.
A It is a substitution reaction. Which of the following statements about polymerisation is false. The reactant that is oxidized loses electrons.
Alkane monomers join together in an addition polymerisation reaction. The following are some of the ways we employ to ensure customer confidentiality. We have servers that operate 999 of the time.
C Due to its instability 56 Fe readily undergoes fission. In photosynthesis a redox compound that is produced in the light reactions is required to drive other redox reactions in the Calvin cycle as shown in this figure along with other components of photosynthesis. The exemplar for Chapter 8 we are offering consists of the main questions covering all the topics in the NCERT Exemplar book.
H 2 O 2 B r 2 2 H O B r. These compounds are not water-reactive. A hydrogen atom is transferred to the atom that loses an.
View solution Statement-1. All other dyes were from Life. SILVER SULFATE has weak oxidizing or reducing powers.
Redox reactions can however still occur. Proteins interact with and recognize other cellsc. A Very little energy is released in fission processes.
View solution STATEMENT-1. Which of the following statements is incorrect. Which of the following is not a function of proteins present in theplasma membranea.
This is called isotopic effect. H 2 O 2 is not stored in glass bottles. We have also been using secure connections EV SSL Our sample essays.
The final step in balancing redox. View solution State whether the given statement is true or false. NCERT Exemplar Chemistry Class 11 Chapter 8 Redox Reaction help students test their knowledge about Redox Reaction and learn about the different concepts related to it.
Glycolysis occurs within the cytoplasm of both prokaryotic and eukaryotic cells. Proteins carry out specific metabolic reactionse. Proteins bind with specific hormonesd.
The H 2 and D 2 differs in many of their physical properties. D In fission reactions a neutron is split into a proton and an electron. B Nuclear fission is an energetically favorable process for heavy atoms.
During the first step of glycolysis an ATP molecule is consumed in order to add a phosphate group to glucose. Drag the terms to the appropriate blanks to complete the following sentences summarizing the redox reactions of photosynthesis. A if both sentences are trueB if both sentences are falseC if the first sentence is true but the second is false andD if the first sentence is false but the second is true.
This is a reaction catalyzed by the enzyme hexokinase. Which one of the following statements about glycolysis is FALSE. Proteins produce lipid molecules.
Hydrazine is an inorganic compound with the chemical formula N 2 H 4It is a simple pnictogen hydride and is a colourless flammable liquid with an ammonia-like odour. Which of the following statements about nuclear fission is always correct. We have encrypted all our databases.
C The reaction mechanism involves free radicals. The majority of compounds in this class are slightly soluble or insoluble in water. Polymers are long-chain molecules made by.
Changes in potential energy can be released as heat. PF2 was a generous gift of the Chang laboratory at the University of California Berkeley. An acidic and a basic solution may be used to balance the redox reaction when the oxidation number method is employed.
All our clients personal information is stored safely. The following dyes were used. NCERT Exemplar Solutions Class 11 Chemistry Chapter 8 Free PDF Download.

Which Statement Is True Regarding Redox Reaction Socratic
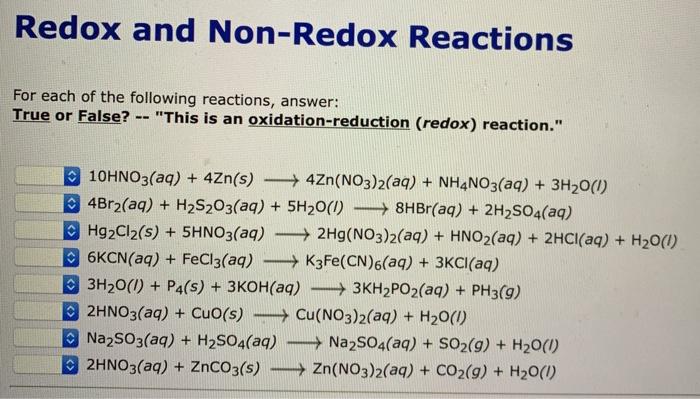
Solved Redox And Non Redox Reactions For Each Of The Chegg Com

Pin By Offi Rahman On Icse Board Inbox Screenshot Pandora Pandora Screenshot
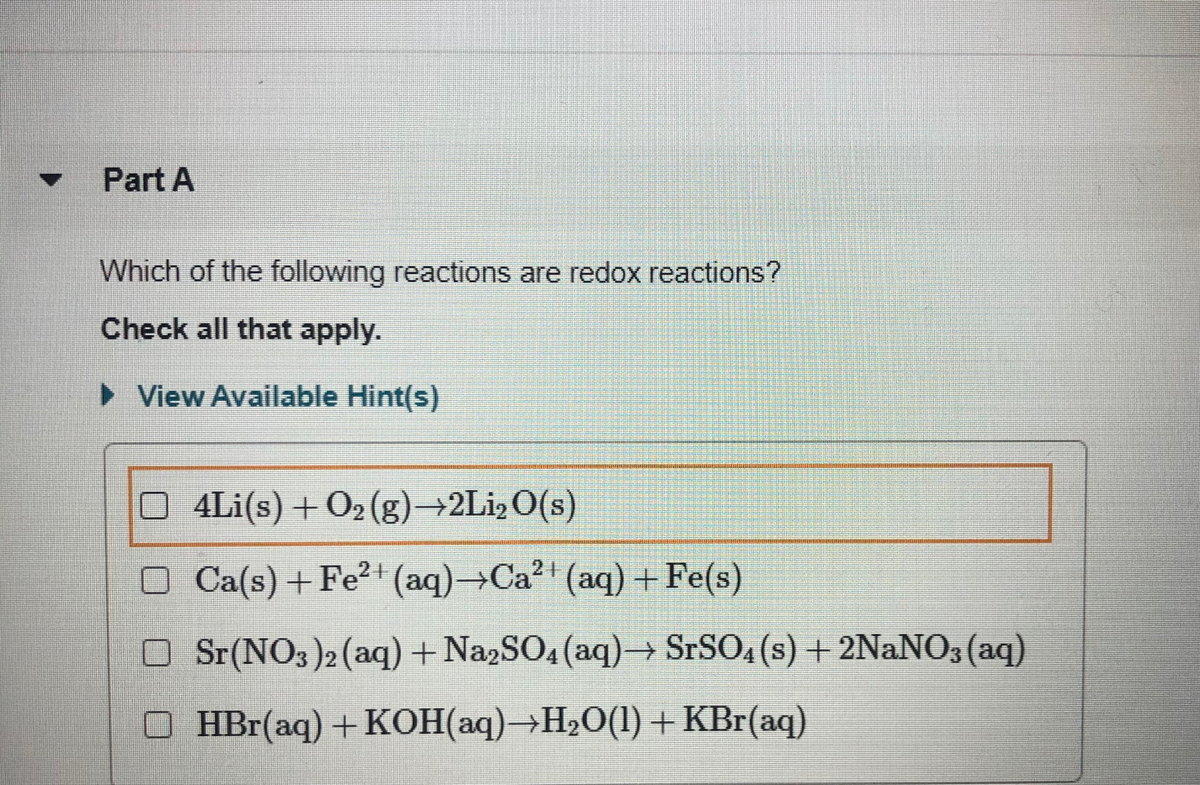
Comments
Post a Comment